What Will Happen to the Ph Inside a Thylakoid That Is Exposed to Light?
Photosynthesis
42 The Light-Dependent Reactions of Photosynthesis
Learning Objectives
By the end of this section, yous will be able to practise the post-obit:
- Explain how plants absorb free energy from sunlight
- Describe curt and long wavelengths of light
- Describe how and where photosynthesis takes place within a establish
How tin lite energy be used to make food? When a person turns on a lamp, electric free energy becomes light energy. Like all other forms of kinetic energy, calorie-free tin travel, modify grade, and exist harnessed to practice work. In the case of photosynthesis, light energy is converted into chemic energy, which photoautotrophs use to build basic carbohydrate molecules ((Figure)). Nonetheless, autotrophs only use a few specific wavelengths of sunlight.
Photoautotrophs tin can capture visible light energy in specific wavelengths from the lord's day, converting it into the chemical energy used to build food molecules. (credit: Gerry Atwell)

What Is Low-cal Free energy?
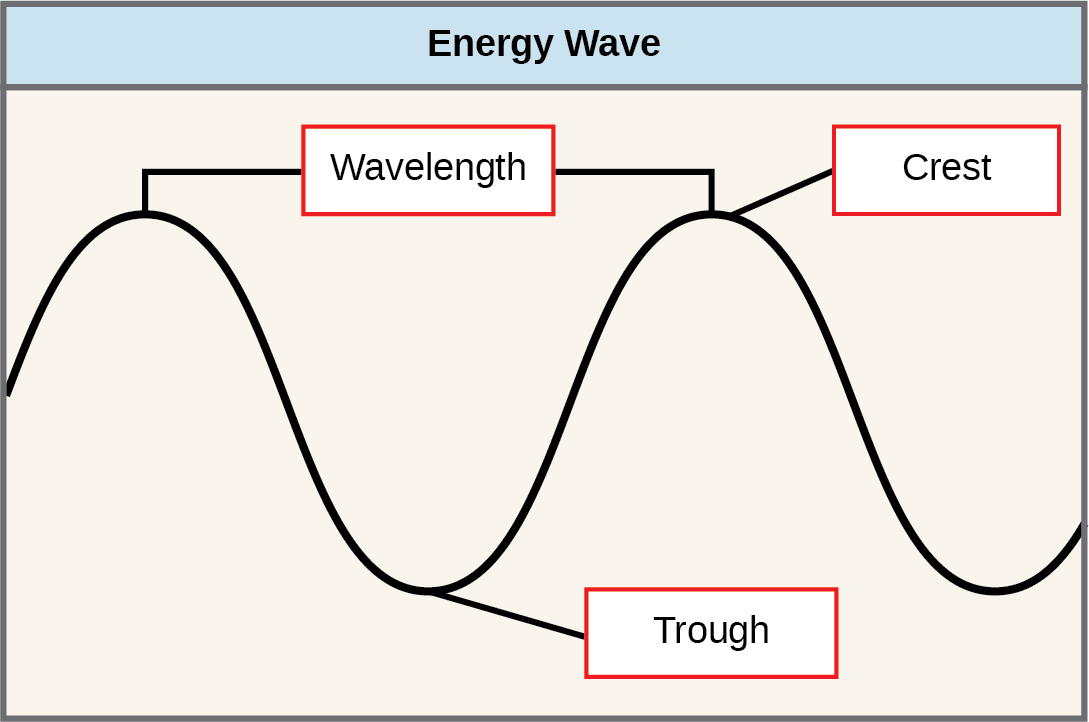
The sun emits an enormous amount of electromagnetic radiations (solar energy in a spectrum from very curt gamma rays to very long radio waves). Humans can come across only a tiny fraction of this energy, which nosotros refer to as "visible light." The manner in which solar free energy travels is described equally waves. Scientists can determine the amount of energy of a moving ridge by measuring its wavelength (shorter wavelengths are more than powerful than longer wavelengths)—the distance betwixt consecutive crest points of a wave. Therefore, a single wave is measured from ii consecutive points, such equally from crest to crest or from trough to trough ((Figure)).
The wavelength of a single wave is the distance between two consecutive points of similar position (two crests or 2 troughs) along the wave.
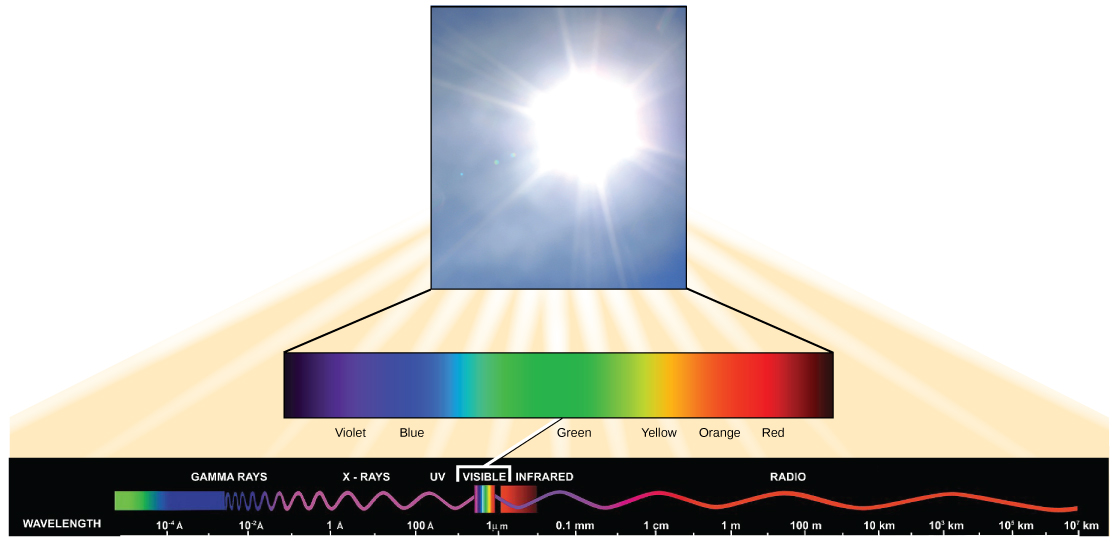
Visible light constitutes only one of many types of electromagnetic radiation emitted from the sun and other stars. Scientists differentiate the various types of radiant energy from the sun within the electromagnetic spectrum. The electromagnetic spectrum is the range of all possible frequencies of radiation ((Figure)). The divergence between wavelengths relates to the amount of energy carried by them.
The sun emits energy in the form of electromagnetic radiation. This radiation exists at unlike wavelengths, each of which has its ain characteristic energy. All electromagnetic radiation, including visible light, is characterized past its wavelength.
Each type of electromagnetic radiation travels at a particular wavelength. The longer the wavelength, the less free energy it carries. Short, tight waves carry the virtually energy. This may seem illogical, but think of it in terms of a slice of moving heavy rope. It takes little effort by a person to move a rope in long, wide waves. To make a rope move in curt, tight waves, a person would demand to apply significantly more energy.
The electromagnetic spectrum ((Effigy)) shows several types of electromagnetic radiation originating from the sun, including 10-rays and ultraviolet (UV) rays. The higher-energy waves tin can penetrate tissues and harm cells and DNA, which explains why both 10-rays and UV rays can be harmful to living organisms.
Assimilation of Light
Light energy initiates the procedure of photosynthesis when pigments blot specific wavelengths of visible light. Organic pigments, whether in the human retina or the chloroplast thylakoid, accept a narrow range of free energy levels that they can absorb. Energy levels lower than those represented by red light are insufficient to raise an orbital electron to a excited (quantum) country. Energy levels college than those in blue light volition physically tear the molecules apart, in a process chosen bleaching. Our retinal pigments can only "see" (absorb) wavelengths between 700 nm and 400 nm of calorie-free, a spectrum that is therefore called visible light. For the same reasons, plants, paint molecules absorb simply calorie-free in the wavelength range of 700 nm to 400 nm; plant physiologists refer to this range for plants as photosynthetically agile radiations.
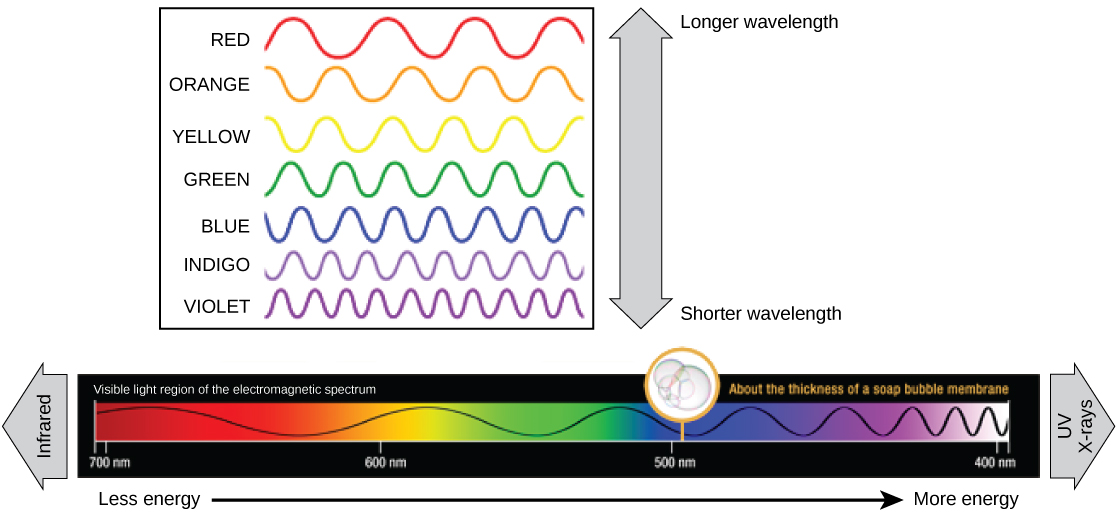
The visible light seen past humans as white light actually exists in a rainbow of colors. Sure objects, such as a prism or a driblet of h2o, disperse white lite to reveal the colors to the man eye. The visible low-cal portion of the electromagnetic spectrum shows the rainbow of colors, with violet and blue having shorter wavelengths, and therefore higher energy. At the other end of the spectrum toward red, the wavelengths are longer and have lower energy ((Figure)).
The colors of visible light do non carry the same amount of energy. Violet has the shortest wavelength and therefore carries the well-nigh free energy, whereas red has the longest wavelength and carries the to the lowest degree amount of energy. (credit: modification of work by NASA)
Agreement Pigments
Unlike kinds of pigments be, and each absorbs but specific wavelengths (colors) of visible light. Pigments reflect or transmit the wavelengths they cannot absorb, making them announced a mixture of the reflected or transmitted low-cal colors.
Chlorophylls and carotenoids are the two major classes of photosynthetic pigments found in plants and algae; each grade has multiple types of pigment molecules. There are v major chlorophylls: a, b, c and d and a related molecule constitute in prokaryotes chosen bacteriochlorophyll. Chlorophyll a and chlorophyll b are found in higher plant chloroplasts and will be the focus of the following word.
With dozens of dissimilar forms, carotenoids are a much larger group of pigments. The carotenoids found in fruit—such every bit the cherry of tomato (lycopene), the yellow of corn seeds (zeaxanthin), or the orange of an orange peel (β-carotene)—are used as advertisements to attract seed dispersers. In photosynthesis, carotenoids function equally photosynthetic pigments that are very efficient molecules for the disposal of backlog free energy. When a leaf is exposed to full sun, the calorie-free-dependent reactions are required to process an enormous amount of energy; if that free energy is non handled properly, it can do pregnant harm. Therefore, many carotenoids reside in the thylakoid membrane, absorb excess energy, and safely misemploy that free energy as heat.
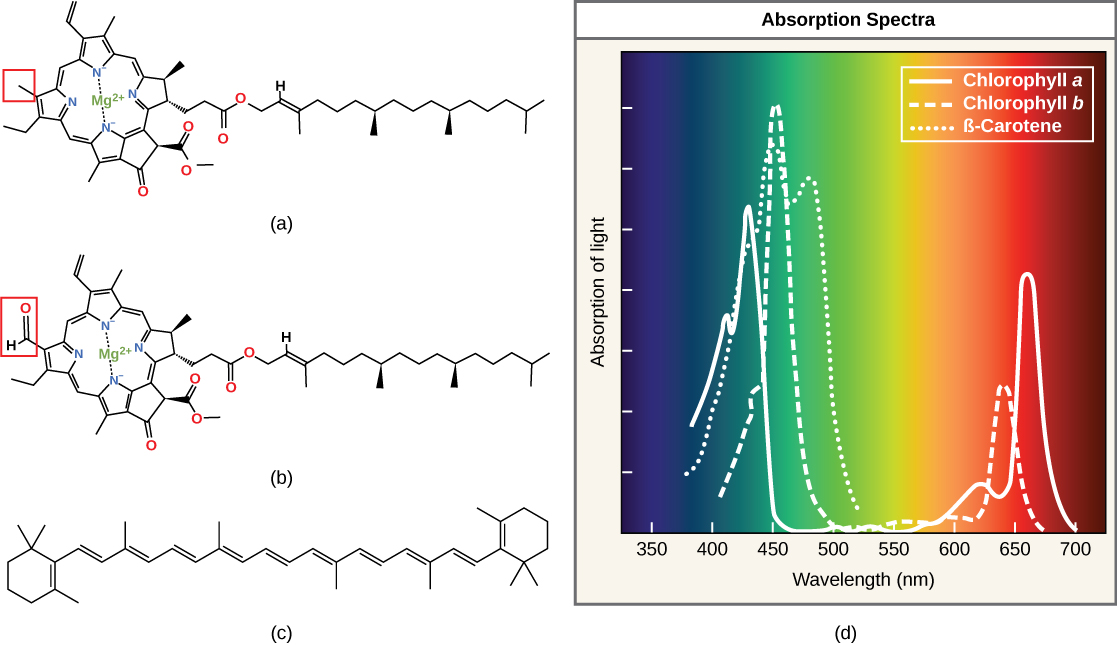
Each type of pigment tin can be identified by the specific pattern of wavelengths it absorbs from visible lite: This is termed the assimilation spectrum. The graph in (Figure) shows the absorption spectra for chlorophyll a, chlorophyll b, and a type of carotenoid pigment called β-carotene (which absorbs blue and green calorie-free). Notice how each pigment has a singled-out set of peaks and troughs, revealing a highly specific blueprint of absorption. Chlorophyll a absorbs wavelengths from either finish of the visible spectrum (blueish and red), simply not green. Because light-green is reflected or transmitted, chlorophyll appears green. Carotenoids absorb in the short-wavelength bluish region, and reflect the longer yellow, reddish, and orange wavelengths.
(a) Chlorophyll a, (b) chlorophyll b, and (c) β-carotene are hydrophobic organic pigments found in the thylakoid membrane. Chlorophyll a and b, which are identical except for the function indicated in the red box, are responsible for the green color of leaves. β-carotene is responsible for the orange colour in carrots. Each pigment has (d) a unique absorbance spectrum.
Many photosynthetic organisms have a mixture of pigments, and by using these pigments, the organism tin can absorb energy from a wider range of wavelengths. Not all photosynthetic organisms have total access to sunlight. Some organisms abound underwater where light intensity and quality decrease and change with depth. Other organisms grow in competition for light. Plants on the rainforest flooring must be able to absorb any bit of low-cal that comes through, considering the taller trees absorb most of the sunlight and besprinkle the remaining solar radiation ((Figure)).
Plants that normally grow in the shade have adapted to depression levels of light by changing the relative concentrations of their chlorophyll pigments. (credit: Jason Hollinger)

When studying a photosynthetic organism, scientists tin determine the types of pigments present by generating assimilation spectra. An instrument chosen a spectrophotometer can differentiate which wavelengths of light a substance tin can absorb. Spectrophotometers measure transmitted calorie-free and compute from it the absorption. By extracting pigments from leaves and placing these samples into a spectrophotometer, scientists tin place which wavelengths of light an organism can absorb. Additional methods for the identification of constitute pigments include various types of chromatography that dissever the pigments by their relative affinities to solid and mobile phases.
How Light-Dependent Reactions Piece of work
The overall role of calorie-free-dependent reactions is to convert solar free energy into chemical energy in the form of NADPH and ATP. This chemic energy supports the light-independent reactions and fuels the assembly of sugar molecules. The light-dependent reactions are depicted in (Figure). Protein complexes and pigment molecules piece of work together to produce NADPH and ATP. The numbering of the photosystems is derived from the guild in which they were discovered, not in the order of the transfer of electrons.
A photosystem consists of i) a light-harvesting complex and two) a reaction center. Pigments in the light-harvesting complex pass light free energy to two special chlorophyll a molecules in the reaction middle. The light excites an electron from the chlorophyll a pair, which passes to the primary electron acceptor. The excited electron must and then be replaced. In (a) photosystem II, the electron comes from the splitting of water, which releases oxygen as a waste material. In (b) photosystem I, the electron comes from the chloroplast electron send chain discussed below.
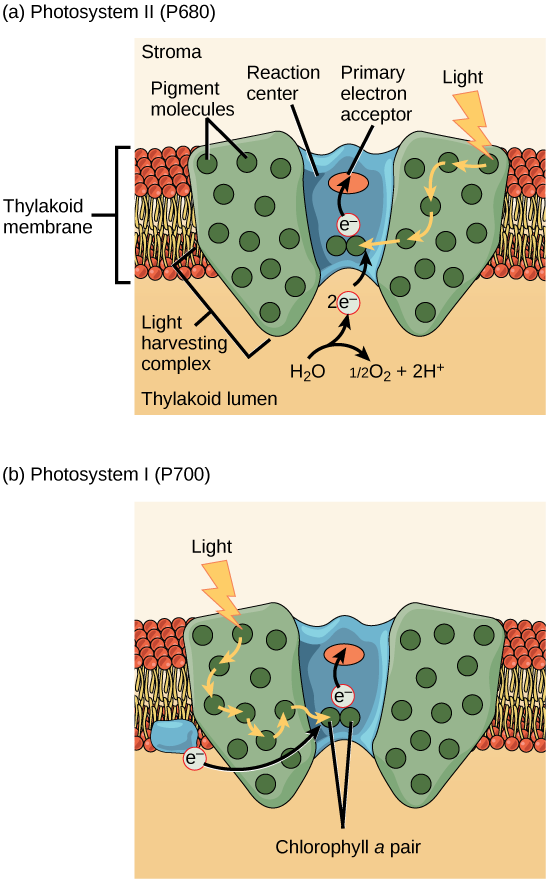
The bodily step that converts light energy into chemical energy takes place in a multiprotein circuitous chosen a photosystem, ii types of which are constitute embedded in the thylakoid membrane: photosystem Two (PSII) and photosystem I (PSI) ((Figure)). The 2 complexes differ on the basis of what they oxidize (that is, the source of the low-energy electron supply) and what they reduce (the place to which they deliver their energized electrons).
Both photosystems have the same basic structure; a number of antenna proteins to which the chlorophyll molecules are bound surround the reaction centre where the photochemistry takes place. Each photosystem is serviced by the lite-harvesting complex, which passes energy from sunlight to the reaction middle; it consists of multiple antenna proteins that incorporate a mixture of 300 to 400 chlorophyll a and b molecules as well as other pigments like carotenoids. The assimilation of a unmarried photon or distinct quantity or "bundle" of light by whatsoever of the chlorophylls pushes that molecule into an excited state. In short, the lite energy has at present been captured past biological molecules just is non stored in whatever useful form yet. The energy is transferred from chlorophyll to chlorophyll until eventually (after about a millionth of a second), information technology is delivered to the reaction center. Upwards to this point, only energy has been transferred between molecules, not electrons.
Visual Connection
In the photosystem II (PSII) reaction centre, free energy from sunlight is used to excerpt electrons from water. The electrons travel through the chloroplast electron transport concatenation to photosystem I (PSI), which reduces NADP+ to NADPH. The electron transport chain moves protons across the thylakoid membrane into the lumen. At the same time, splitting of water adds protons to the lumen, and reduction of NADPH removes protons from the stroma. The net effect is a low pH in the thylakoid lumen, and a high pH in the stroma. ATP synthase uses this electrochemical slope to make ATP.
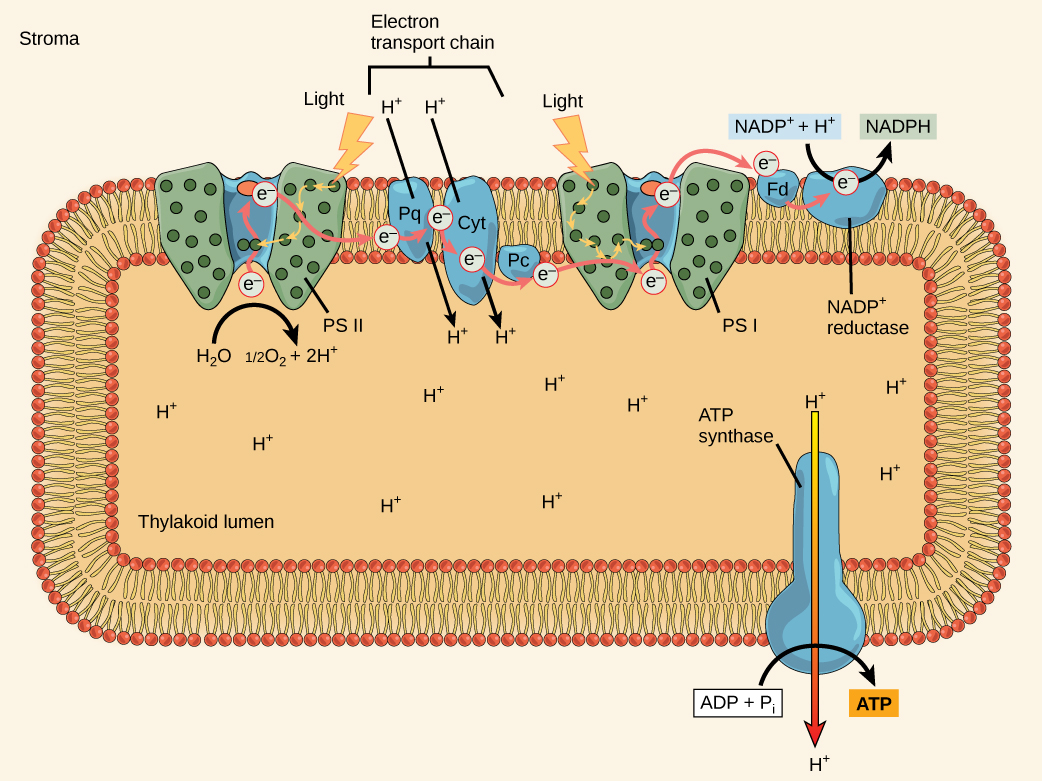
What is the initial source of electrons for the chloroplast electron send concatenation?
- water
- oxygen
- carbon dioxide
- NADPH
<!– <para> A –>
The reaction center contains a pair of chlorophyll a molecules with a special property. Those two chlorophylls can undergo oxidation upon excitation; they can actually give upwards an electron in a process called a photoact. It is at this step in the reaction center during photosynthesis that light energy is converted into an excited electron. All of the subsequent steps involve getting that electron onto the free energy carrier NADPH for delivery to the Calvin cycle where the electron is deposited onto carbon for long-term storage in the course of a carbohydrate. PSII and PSI are 2 major components of the photosynthetic electron send chain, which also includes the cytochrome complex. The cytochrome circuitous, an enzyme equanimous of two protein complexes, transfers the electrons from the carrier molecule plastoquinone (Pq) to the protein plastocyanin (Pc), thus enabling both the transfer of protons across the thylakoid membrane and the transfer of electrons from PSII to PSI.
The reaction center of PSII (called P680) delivers its loftier-energy electrons, one at the time, to the main electron acceptor, and through the electron transport chain (Pq to cytochrome complex to plastocyanine) to PSI. P680'due south missing electron is replaced by extracting a low-energy electron from water; thus, h2o is "split up" during this stage of photosynthesis, and PSII is re-reduced afterward every photoact. Splitting i H2O molecule releases 2 electrons, two hydrogen atoms, and one atom of oxygen. Still, splitting ii molecules is required to form one molecule of diatomic O2 gas. About 10 percent of the oxygen is used past mitochondria in the leaf to support oxidative phosphorylation. The remainder escapes to the atmosphere where information technology is used by aerobic organisms to support respiration.
As electrons movement through the proteins that reside between PSII and PSI, they lose energy. This free energy is used to motility hydrogen atoms from the stromal side of the membrane to the thylakoid lumen. Those hydrogen atoms, plus the ones produced by splitting h2o, accrue in the thylakoid lumen and volition be used synthesize ATP in a afterwards step. Because the electrons accept lost free energy prior to their arrival at PSI, they must exist re-energized by PSI, hence, another photon is captivated by the PSI antenna. That energy is relayed to the PSI reaction center (chosen P700). P700 is oxidized and sends a loftier-energy electron to NADP+ to course NADPH. Thus, PSII captures the free energy to create proton gradients to brand ATP, and PSI captures the energy to reduce NADP+ into NADPH. The two photosystems work in concert, in part, to guarantee that the production of NADPH will roughly equal the production of ATP. Other mechanisms exist to fine-tune that ratio to exactly match the chloroplast'due south constantly changing energy needs.
Generating an Energy Carrier: ATP
As in the intermembrane infinite of the mitochondria during cellular respiration, the buildup of hydrogen ions inside the thylakoid lumen creates a concentration gradient. The passive diffusion of hydrogen ions from high concentration (in the thylakoid lumen) to low concentration (in the stroma) is harnessed to create ATP, simply every bit in the electron send chain of cellular respiration. The ions build up free energy because of improvidence and because they all take the same electrical charge, repelling each other.
To release this energy, hydrogen ions will rush through whatsoever opening, similar to water jetting through a hole in a dam. In the thylakoid, that opening is a passage through a specialized protein channel called the ATP synthase. The free energy released by the hydrogen ion stream allows ATP synthase to attach a third phosphate grouping to ADP, which forms a molecule of ATP ((Figure)). The flow of hydrogen ions through ATP synthase is called chemiosmosis because the ions move from an area of high to an expanse of depression concentration through a semi-permeable structure of the thylakoid.
Link to Learning
Visit this site and click through the animation to view the procedure of photosynthesis inside a foliage.
Section Summary
The pigments of the get-go office of photosynthesis, the light-dependent reactions, blot energy from sunlight. A photon strikes the antenna pigments of photosystem Two to initiate photosynthesis. The energy travels to the reaction middle that contains chlorophyll a and then to the electron transport concatenation, which pumps hydrogen ions into the thylakoid interior. This activity builds up a high concentration of hydrogen ions. The hydrogen ions menses through ATP synthase during chemiosmosis to form molecules of ATP, which are used for the formation of saccharide molecules in the 2d stage of photosynthesis. Photosystem I absorbs a second photon, which results in the germination of an NADPH molecule, another energy and reducing carrier for the light-independent reactions.
Visual Connexion Questions
(Figure) What is the source of electrons for the chloroplast electron transport concatenation?
- Water
- Oxygen
- Carbon dioxide
- NADPH
(Figure) A.
Review Questions
Which of the following structures is non a component of a photosystem?
- ATP synthase
- antenna molecule
- reaction centre
- chief electron acceptor
A
How many photons does it accept to fully reduce one molecule of NADP+ to NADPH?
- 1
- two
- four
- 8
B
Which complex is not involved in the institution of atmospheric condition for ATP synthesis?
- photosystem I
- ATP synthase
- photosystem II
- cytochrome complex
C
From which component of the light-dependent reactions does NADPH form most directly?
- photosystem 2
- photosystem I
- cytochrome circuitous
- ATP synthase
B
Three of the aforementioned species of institute are each grown under a different colored light for the same corporeality of fourth dimension. Plant A is grown under blue light, Institute B is grown under light-green light, and Establish C is grown nether orange lite. Bold the plants employ simply chlorophyll a and chlorophyll b for photosynthesis, what would be the predicted order of the plants from most growth to least growth?
- A, C, B
- A, B, C
- C, A, B
- B, A, C
A
Plants containing only chlorophyll b are exposed to radiation with the post-obit wavelengths: 10nm (x-rays), 450nm (blue low-cal), 670nm (carmine light), and 800nm (infrared light). Which plants harness the nearly free energy for photosynthesis?
- X-ray irradiated plants
- Blue light irradiated plants
- Red light irradiated plants
- Infrared irradiated plants
B
Critical Thinking Questions
Describe the pathway of electron transfer from photosystem 2 to photosystem I in light-dependent reactions.
A photon of light hits an antenna molecule in photosystem II, and the energy released by information technology travels through other antenna molecules to the reaction center. The free energy causes an electron to leave a molecule of chlorophyll a to a primary electron acceptor poly peptide. The electron travels through the electron ship chain and is accustomed by a pigment molecule in photosystem I.
What are the roles of ATP and NADPH in photosynthesis?
Both of these molecules carry energy; in the case of NADPH, it has reducing ability that is used to fuel the process of making carbohydrate molecules in calorie-free-independent reactions.
How and why would the end products of photosynthesis be changed if a found had a mutation that eliminated its photosystem II complex?
Knocking out photosystem Ii would eliminate the production of oxygen and ATP during photosynthesis. Photosystem II splits water into oxygen atoms, hydrogen protons that remain in the thylakoid lumen, and hydrogen-derived electrons that motion from the reaction center into the electron transport chain. The transfer of an electron through the electron transport concatenation provides the energy to pump more protons into the thylakoid lumen to maintain a higher concentration of protons there. Moving protons across the thylakoid membrane back to the stroma provides the energy for ATP synthase to produce ATP. Without this proton gradient, ATP volition not be synthesized.
Glossary
- absorption spectrum
- range of wavelengths of electromagnetic radiation absorbed by a given substance
- antenna protein
- pigment molecule that directly absorbs low-cal and transfers the energy absorbed to other pigment molecules
- carotenoid
- photosynthetic pigment (yellowish-orange-reddish) that functions to dispose of excess free energy
- chlorophyll a
- course of chlorophyll that absorbs violet-blue and red lite and consequently has a bluish-green colour; the simply paint molecule that performs the photochemistry by getting excited and losing an electron to the electron transport chain
- chlorophyll b
- accessory pigment that absorbs blueish and red-orange calorie-free and consequently has a yellowish-green tint
- cytochrome complex
- group of reversibly oxidizable and reducible proteins that forms part of the electron transport concatenation between photosystem II and photosystem I
- electromagnetic spectrum
- range of all possible frequencies of radiation
- electron transport chain
- group of proteins between PSII and PSI that pass energized electrons and employ the energy released past the electrons to motility hydrogen ions against their concentration slope into the thylakoid lumen
- light harvesting complex
- circuitous that passes energy from sunlight to the reaction center in each photosystem; it consists of multiple antenna proteins that contain a mixture of 300 to 400 chlorophyll a and b molecules besides as other pigments like carotenoids
- P680
- reaction centre of photosystem II
- P700
- reaction heart of photosystem I
- photoact
- ejection of an electron from a reaction center using the energy of an absorbed photon
- photon
- distinct quantity or "parcel" of light energy
- photosystem
- grouping of proteins, chlorophyll, and other pigments that are used in the light-dependent reactions of photosynthesis to absorb light energy and convert it into chemical free energy
- photosystem I
- integral pigment and protein complex in thylakoid membranes that uses light energy to transport electrons from plastocyanin to NADP+ (which becomes reduced to NADPH in the process)
- photosystem 2
- integral protein and pigment complex in thylakoid membranes that transports electrons from water to the electron transport concatenation; oxygen is a production of PSII
- main electron acceptor
- pigment or other organic molecule in the reaction heart that accepts an energized electron from the reaction center
- reaction center
- complex of chlorophyll molecules and other organic molecules that is assembled around a special pair of chlorophyll molecules and a main electron acceptor; capable of undergoing oxidation and reduction
- spectrophotometer
- instrument that can mensurate transmitted calorie-free and compute the assimilation
- wavelength
- distance between consecutive points of equal position (two crests or two troughs) of a wave in a graphic representation; inversely proportional to the energy of the radiation
Source: https://opentextbc.ca/biology2eopenstax/chapter/the-light-dependent-reactions-of-photosynthesis/
0 Response to "What Will Happen to the Ph Inside a Thylakoid That Is Exposed to Light?"
Post a Comment